Basic Terms:
Kinematics: The analysis of the motion of an object without considering the forces acting on it
Dynamics: The study of the motion of an object with considerations of the object's mass and the forces acting on it
Mechanics: The study of the motions with considerations of both kinematics and dynamics.
Scalar Quantity: Any quantity that can be defined by a single number and a unit of measure (e.g., mass, distance, speed)
Vector Quantity: Any quantity that must be defined by using a number, a unit of measure, and a direction (e.g., velocity, which you have to specify the direction).
Position: The location of an object with respect to the reference point. It's a vector quantity.
Tuesday, January 24, 2012
Monday, January 23, 2012
Naming Common Functional Groups
Alcohol:
For IUPAC naming, there are two ways that you may name an alcohol:
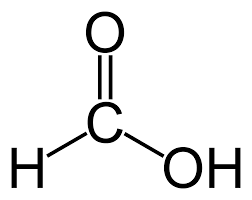
For IUPAC naming, there are two ways that you may name an alcohol:
- Using the -ol ending. For example, if you have a propane that has a hydroxide attached to the middle, then you name it as propan-2-ol:
- Using the n-hydroxy- prefix, where n specifies the location of the hydroxyl group. For example, for the same molecule mentioned above, you would name it as 2-hydroxypropane
Common Naming: methyl alcohol, ethyl alcohol, and propyl alcohol
Carboxylic Acids:
IUPAC: Whenever you see a carboxylic acid, it must be included as part of the main chain, and the carbon that has the double-bond-O must be numbered as 1.
Then, what you do is that you name the rest of the molecule normally, and then add -oic acid to the end of the name. For example, the following would be named as methanoic acid:
Common Naming: formic acid, acetic acid, and proprionic acid.
Labels:
Chemistry
Chemical Equilibrium #5: Steps for Solving an Equilibrium Question
Although equilibrium questions are generally straight-forward, they require a lot of steps, and as a result it is very easy to make a mistake while solving them.
So I have come up with a step-by-step procedure to ensure that the solution is 100% error-free:
So I have come up with a step-by-step procedure to ensure that the solution is 100% error-free:
- Balance the chemical equation(s)
- Set up an ICE table: make sure that all values are filled into the appropriate spaces and that the variables all have correct coefficients. Also make sure that all values have the correct number of decimal places
- Write out the equilibrium law and solve: make sure that each reactant has the correct exponent
- Plug the answer back into the equilibrium law, and make sure that all the values match with those of the question
- Look at your answer, and see if it seems strange. If so, (e.g., a pH of 6.8 for an acid), repeat all the steps
Labels:
Chemistry
Sunday, January 22, 2012
Types of Organic Reactions
Five Types: Addition, Elimination, Subtraction, Condensation, and Oxidation
Addition Reactions: Reactions where a double bond is broken down into a single bond by the addition of two groups of atoms.

Elimination Reactions:
The reverse of addition reactions.
Substitution Reactions: an atom or side chain is replaced by another atom or side chain.

Condensation Reactions: new functional groups are synthesized as a result of the release of a water molecule

Oxidation Reactions: Reactions where a hydrogen atom is lost or an oxygen is gained:

Addition Reactions: Reactions where a double bond is broken down into a single bond by the addition of two groups of atoms.
- Hydrogenation: two hydrogen atoms are added (H-H)
- Hydrohalogenation: one hydrogen and one halogen is added (H-Cl)
- Hydration: one hydrogen and one hydroxyl is added
- Halogenation: two halogen atoms (Cl- Cl)

Elimination Reactions:
The reverse of addition reactions.
Substitution Reactions: an atom or side chain is replaced by another atom or side chain.
- Mostly aromatic compounds
- Amines can be prepared by substituting the halogen of an alkylhalide by an ammonia

Condensation Reactions: new functional groups are synthesized as a result of the release of a water molecule
- alcohol + alcohol→ ether + water
- alcohol + carboxylic acid → ester + water
- amine + carboxylic acid → amide + water

Oxidation Reactions: Reactions where a hydrogen atom is lost or an oxygen is gained:
- Alcohols that occur in the middle of a carbon chain can oxidize to form ketones. (the H of the Alcohol is lost and a double bond results)
- Alcohols that occur at the end of a carbon chain can oxidize to form aldehydes. (the H of the Alcohol is lost and a double bond results)
- Aldehydes can further oxidize into carboxylic acides (an oxygen atom can be gained, forming an OH group)

Labels:
Chemistry
Sinusoidol Functions:Transformations
This is another brief post for my exam studying.
Like most other kinds of functions, sinusoidal functions can can have transformations applied on their graphs:
Like most other kinds of functions, sinusoidal functions can can have transformations applied on their graphs:
f(x) = a sin (k(x-d))+ c
a is the vertical stretch and/or reflection. |a| is the amplitude.
k is the horizontal stretch and/or reflection, and it affects the period of the function. The period is |360÷k|
d is the horizontal translation. It doesn't affect the properties of the function
c is the vertical translation, and it determines what the equation of the axis is: the equation of axis is y=c
Also, using a and c, one can find the maximum and minimum points on the graph:
- maximum value= c+ |a|
- minimum value = c- |a|
Labels:
math
Rates of Reaction #4: Reaction mechanisms
Sometimes, a reaction may not just occur in one single step. Instead, it may take place over a series of steps. For example, if X, Y and Z were to react, X and Y may first form a reaction intermediate, then that reaction intermediate may then react with Z to form the final products.
Each single step in an overall reaction is called an elementary step, and the series of elementary steps is called a reaction mechanism.
The slowest elementary process is called the rate-determining step, and its rate law is the rate law for the overall reaction.
Each single step in an overall reaction is called an elementary step, and the series of elementary steps is called a reaction mechanism.
The slowest elementary process is called the rate-determining step, and its rate law is the rate law for the overall reaction.
Labels:
Chemistry
Rates of Reaction #3: Rate Theories
Collision theory:
- Reaction rate is directly proportional to the number of successful collisions per second
- Factors that effect the success of a collision:
- Orientation: Molecules have to collide at certain angles in order for them to react
- Kinetic Energy: When molecules collide, they must have enough kinetic energy in order for them to undergo reaction
Transition State Theory
Transition state complexes occur at the peaks of the potential energy graphs, and they are large molecules made up of all the reactants. Bonds are forming and breaking at the same time.
Catalysts can reduce the activation energy by one or more of the following mechanisms:
- bend or stretch bonds to make them easier to break
- bring two reactants closer together
- provide a different reaction
- provide a microenvironment
Labels:
Chemistry
Saturday, January 21, 2012
Rates of Reaction #2: The Rate Law
The rate law for any chemical reaction is:
where:
Order of reaction: sum of the rate law exponents
Half Life:
For some radioactive decay, the concentration of a substance is halved consistently after a certain duration of time. For example, some radioactive material's concentration may be halved every four hours.
The time that it takes for such a substance to decrease by half is called half life, or t½ . Andinterestingly, this can be used to determine the rate constant:
r=k[A]x[B]y
where:
- k is the rate constant, dependent on the temperature, surface area, etc.
- [A] and [B] are the concentrations of the reactants
- x and y are values determined by experiments.
Order of reaction: sum of the rate law exponents
Half Life:
For some radioactive decay, the concentration of a substance is halved consistently after a certain duration of time. For example, some radioactive material's concentration may be halved every four hours.
The time that it takes for such a substance to decrease by half is called half life, or t½ . And
k=ln(2) ÷ t½
Labels:
Chemistry
Rates of Reaction #1: Factors Affecting Reaction Rates
1. Chemical Natures of Reactants
Depending on the natures of the substances that are reacted, reaction rates may vary. For example, acid/base reactions usually occur quickly, where as oxidation of iron occurs over a long period of time
2. Surface Area
For heterogeneous reactions— reactions where the reactants are in different states— reaction can only occur at the surface between the two states. Therefore, the larger the surface area, the faster the reaction rate.
3. Concentration
According to the collision theory, reactions can only occur when molecules collide. If the concentrations of the reactants are higher, there will be more collisions, and thus the reaction rate will increase. It is one of the most common methods of changing reaction rates.
4. Temperature
At a greater temperature, molecules will have more kinetic energy. This not only means that collisions will be more frequent, but also that more molecules will have sufficient energy to reactant when they collide
5. Catalyst
A catalyst changes the reaction mechanism such that the activation energy (energy required for the molecules to react) is lower. This means that more molecules will have sufficient energy to react when they collide.
Depending on the natures of the substances that are reacted, reaction rates may vary. For example, acid/base reactions usually occur quickly, where as oxidation of iron occurs over a long period of time
2. Surface Area
For heterogeneous reactions— reactions where the reactants are in different states— reaction can only occur at the surface between the two states. Therefore, the larger the surface area, the faster the reaction rate.
3. Concentration
According to the collision theory, reactions can only occur when molecules collide. If the concentrations of the reactants are higher, there will be more collisions, and thus the reaction rate will increase. It is one of the most common methods of changing reaction rates.
4. Temperature
At a greater temperature, molecules will have more kinetic energy. This not only means that collisions will be more frequent, but also that more molecules will have sufficient energy to reactant when they collide
5. Catalyst
A catalyst changes the reaction mechanism such that the activation energy (energy required for the molecules to react) is lower. This means that more molecules will have sufficient energy to react when they collide.
Labels:
Chemistry
Thermodynamics #3: Nuclear Reactions

Fusion: smaller nuclei are joined together to form larger nuclei
Fission: larger nuclei are broken into smaller nuclei by bombardment of a slow-moving neutron
Since both types of nuclear reactions release a lot of energy, it is obvious that they have negative enthalpy changes.
Labels:
Chemistry
Thermodynamics #2: Enthalpy
Enthalpy:
- Enthalpy (H): the total amount of kinetic and potential energy stored in a chemical system
- Change in enthalpy (ΔH): the change in enthalpy when a system undergoes a reaction
- Standard Enthalpy (ΔH°): the enthalpy change for a balanced chemical reaction
- Standard Enthalpy of Formation (ΔH°f ): the enthalpy of a reaction when one mole of a particular substance is formed at SATP from its constituent elements at their standard states
- Enthalpy of combustion (ΔH°c ): the enthalpy for the combustion of one mole of a compound
Labels:
Chemistry
Thermodynamics #1: Heat Capacity and Calorimetry
Basic Terms
Heat: transfer of energy due to contact
Thermal energy: the amount of energy in an object directly related to temperature
Temperature: the measure of internal energy of an object due to particle motion
Heat Capacities
Specific heat capacity: amount of heat needed to raise the temperature of one gram of a substance by 1 degree Celsius
Heat capacity: amount of heat needed to raise the temperature of one gram of a sample by 1 degree Celsius
Calorimetry: The science of measuring the heat of chemical reactions.
| Usually uses an insulator, like styrofoam, to prevent heat loss |
Types of Systems
Open: a system where both matter and energy may be transferred between the system and the surroundings
Closed: a system where energy may be transferred between the system and the surroundings
Isolated: a system where neither matter nor energy can be transferred between the system and the surrounding
Labels:
Chemistry
Magnetism: Right Hand Rule #4
Brief History
- In 1820, Ampere showed that a constant current could produce a constant magnetic field
- In 1832, Michael Faraday tried to prove that a constant magnetic field could produce a constant current. However, during his experiment, he found out that it was only when the magnetic filed was changing that current was produced. This was how alternating current (AC) was discovered.
General effect
Moving a magnet toward a copper ring will induce a current in the copper ring. However, once the movement is stopped, the current will disappear
Right Hand Rule #4
(Unfortunately, I do not have enough time to make a picture)
With an open palm:
- Thumb points toward the relative motion of the copper ring or coil (the magnet is the reference point).
- Fingers point toward the direction of the magnetic field has it crosses the copper ring
- Out of the palm is the direction of the current
Lenz's Law
Using the law of energy conservation, Heinrich Lenz showed that the induced current produced would always produce a magnetic field that would oppose the motion of the coil. So for example, if the north pole of a magnet approaches a coil, this is what would happen:
Labels:
physics
Magnetism: Right Hand Rule #3
Background information
If a current-carrying conductor is placed perpendicular to the magnetic field of a magnet, there will be a force acting on the conductor.
Right Hand Rule #3
Parallel conductors
When two current-carrying conductors are parallel to each other, they would either attract or repel each other. If the two currents are in the same direction, the two conductors will attract; if the two currents are in opposite directions, the two conductors will repel.
Motor Force
The force that a magnet exerts on a current-carrying conductor can be found using the following equation:
B is the strength of the magnetic field that is perpendicular to the current
I is the current in amperes
L is the length of the conductor (calculated by number of coils multiplied by the length of each coil)
If a current-carrying conductor is placed perpendicular to the magnetic field of a magnet, there will be a force acting on the conductor.
Right Hand Rule #3
Parallel conductors
When two current-carrying conductors are parallel to each other, they would either attract or repel each other. If the two currents are in the same direction, the two conductors will attract; if the two currents are in opposite directions, the two conductors will repel.
Motor Force
The force that a magnet exerts on a current-carrying conductor can be found using the following equation:
B is the strength of the magnetic field that is perpendicular to the current
I is the current in amperes
L is the length of the conductor (calculated by number of coils multiplied by the length of each coil)
Labels:
physics
Magnetism: Right Hand Rule #2
Term(s):
Solenoid: a coil wound into a tightly packed helix (usually made of copper).
Electromagnet: a type of magnet whose magnetic field is produced by the flow of electric current
Right Hand Rule #2:
Used to find the direction of the north pole when the solenoid is turned on (i.e., when there's current passing through it)
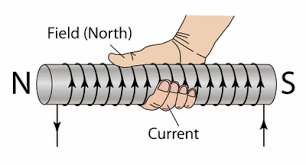
The Electromagnet
When a core of ferromagnetic material, such as iron, is placed inside a solenoid, an electromagnet would be formed. The magnetic field of the solenoid would align the domains in the ferromagnetic material, and the ferromagnetic material would then produce a magnetic field of its own, which supplements the pre-existing magnetic field of the solenoid.
Other Notes:
The magnetic field of a solenoid is similar to that of a bar magnet
Solenoid: a coil wound into a tightly packed helix (usually made of copper).
Electromagnet: a type of magnet whose magnetic field is produced by the flow of electric current
Right Hand Rule #2:
Used to find the direction of the north pole when the solenoid is turned on (i.e., when there's current passing through it)
The Electromagnet
When a core of ferromagnetic material, such as iron, is placed inside a solenoid, an electromagnet would be formed. The magnetic field of the solenoid would align the domains in the ferromagnetic material, and the ferromagnetic material would then produce a magnetic field of its own, which supplements the pre-existing magnetic field of the solenoid.
Other Notes:
The magnetic field of a solenoid is similar to that of a bar magnet
Labels:
physics
Magnetism: Electromagnetism + Right Hand Rule #1
Terms:
Current: the transfer of positive charge; the opposite of electron flow.
Electromagnetism: the interactions between electric currents and magnetic fields
Background Information
Right hand rule #1
Magnetic Field of a Current-Carrying coil
Current: the transfer of positive charge; the opposite of electron flow.
Electromagnetism: the interactions between electric currents and magnetic fields
Background Information
- In 1819, a Danish physicist named Hans Oersted discovered that a current-carrying conductor caused a magnetic compass to deflect. This led him to be credited for the discovery of electromagnetism.
- Electromagnetic fields can be created by the movement of charges, but not by static charges.
Right hand rule #1
| Thumb points toward the current, fingers encircling the conductor in the direction of the magnetic lines of force |
Magnetic Field Theory
- Magnetic field is the source of magnetic forces
- A current creates a magnetic field around itself
- The strength of the magnetic field is directly related to the strength of the current
Magnetic Field of a Current-Carrying coil
 |
| Arrow is the direction of the current; x's are the magnetic fields coming into the page; dots are the magnetic field coming out of the page |
As shown in the picture, the field inside the loop is stronger than the field outside of the loop. Also, the field is more uniformed inside the loop
Labels:
physics
Magnetism: Natural Magnetism
Important Terms:
Ferromagnetic: adjective used to describe metals that are highly magnetic (such as iron, cobalt, and nickel)
Domain: a small region within a magnetic material which has uniform magnetization
Magnetic Field: region of space that will exert a force on a moving charge or magnetic field that enters the space
Field Density: how strong a magnet's magnetic field is
Important Theory/Concepts
Domain Theory: Permanent magnets are are ferromagnetic metals that have all of their domains aligned. On the other hand, non-magnetic ferromagnetic materials' domains are not aligned in any way.

Strength of magnetic forces: The force of attraction or repulsion between two permanent magnets is inversely proportional to the distance between the two.
Non-Permanent Magnets: when a permanent magnet is brought near a non-magnetized piece of ferromagnetic material, the former will temporarily align the domains in the latter, thus magnetizing latter. However, once the former is removed, the domains in the latter will removed back to being random.
Magnetic Field: each magnet has a magnetic field, which can be represented by lines of force. The more lines of force that a magnet has, the stronger it is. The arrows in the diagram represent the directions of the force that an object will experience if it is placed on a particular point in the diagram. Also, all lines of force exit from the north pole and enters the south pole.
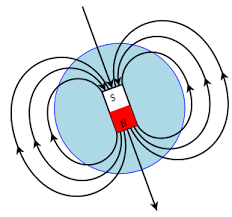
Miscellaneous
Ferromagnetic: adjective used to describe metals that are highly magnetic (such as iron, cobalt, and nickel)
Domain: a small region within a magnetic material which has uniform magnetization
Magnetic Field: region of space that will exert a force on a moving charge or magnetic field that enters the space
Field Density: how strong a magnet's magnetic field is
Important Theory/Concepts
Domain Theory: Permanent magnets are are ferromagnetic metals that have all of their domains aligned. On the other hand, non-magnetic ferromagnetic materials' domains are not aligned in any way.

Strength of magnetic forces: The force of attraction or repulsion between two permanent magnets is inversely proportional to the distance between the two.
Non-Permanent Magnets: when a permanent magnet is brought near a non-magnetized piece of ferromagnetic material, the former will temporarily align the domains in the latter, thus magnetizing latter. However, once the former is removed, the domains in the latter will removed back to being random.
Magnetic Field: each magnet has a magnetic field, which can be represented by lines of force. The more lines of force that a magnet has, the stronger it is. The arrows in the diagram represent the directions of the force that an object will experience if it is placed on a particular point in the diagram. Also, all lines of force exit from the north pole and enters the south pole.
Miscellaneous
- the domains of a magnet become misaligned if the magnet is dropped or hit
- when a permanent magnet is heated above the Curie point (different for each material), it'll lose its magnetic properties
- magnetic fields tend to be strongest at the two poles of the magnet
Labels:
physics
Friday, January 20, 2012
The Picture of Dorian Gray by Oscar Wilde

I don't have much to say about The Picture of Dorian Gray, except that the book is extremely dull and boring, and that it is a book that I would not have finished if it were not for English class.
So how is it boring? Well, it is in many respects. For example, a large part of the book is Lord Henry giving long speeches about hedonism and the pursuit of pleasure; everything he says is quite similar, and it gets repetitive after a short while. Also, when I was reading the novel, I really could not finish reading chapter eleven without falling asleep; the entire chapter merely describes the kinds of music Dorian listens to, the kinds of jewels that he likes, etc.
Labels:
books
Wednesday, January 18, 2012
Exam Time!

Today during third period, I finally had my English Dorian Gray test — my last assessment before the exams. This means that from tomorrow on (since it's already pretty late today), my sole "duty" for school would be to study for the exams.
This semester, I have four exams, meaning that I have one exam for each subject that I have: English, chemistry, math and chemistry. And for the rest of the post, I will just briefly describe each exam that I'll be writing next week.
Monday, January 16, 2012
Oryx and Crake by Margaret Atwood

When I first picked up this Atwood novel in September 2011, I really didn't expect much from it; it was for my English final project, and I only chose it because its description sounded slightly more interesting than the rest of the books that I had to choose from. Also, the fact that it is written by Margaret Atwood actually lowered my expectation from it: if even The Handmaid's Tale— one of Atwood's most renowned and praised works— was boring for me, then how could I expect to like Oryx and Crake, one of Atwood's less popular works?
However, once I started reading this book, I realized that it is a lot better than I had expected. The novel is mysterious, eventful, suspenseful, sad, and thought-provoking—almost everything that one can look for in a good novel.
Labels:
books
Subscribe to:
Posts (Atom)





